
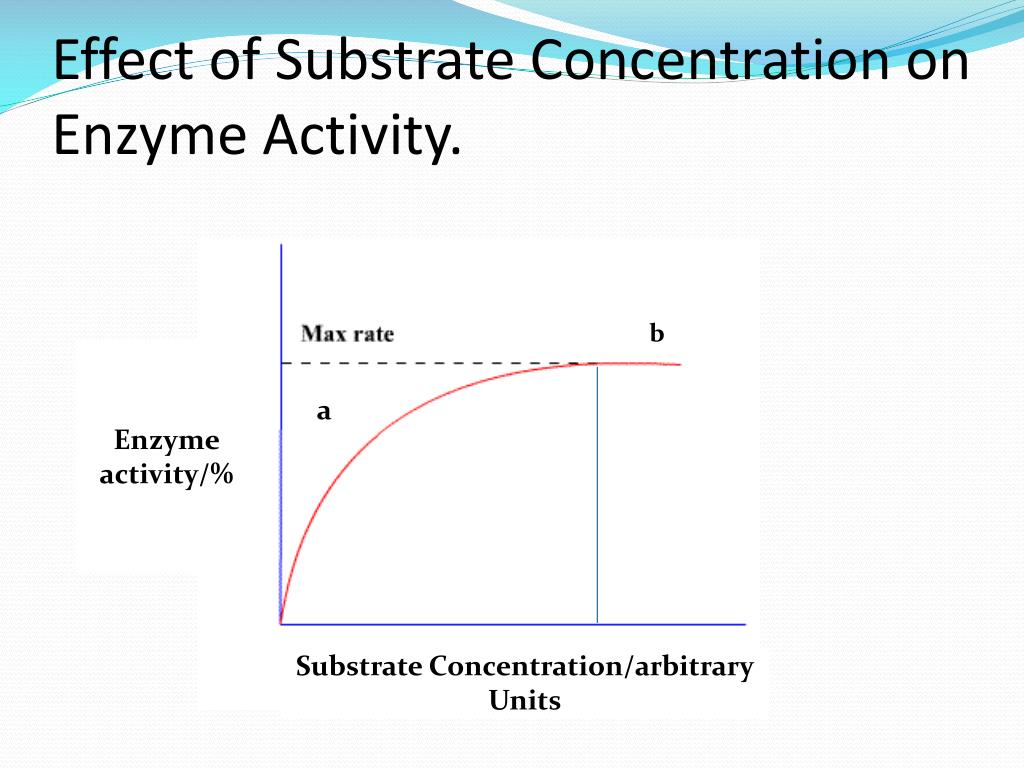
If the substrate is inexpensive, then saturating the reaction with substrate ensures the most product in the shortest period of time. If the substrate is valuable, we can think of K M as the optimal amount of substrate to invest. K M is the substrate concentration midway to the maximum rate, and is a useful value to note since the reaction is non-linear, and return on substrate investment diminishes as we approach the maximum rate (V max). The graph below shows that the rate or velocity (V) of a reaction depends on substrate (K) concentration up to a limit. For example, carbonic anhydrase can catalyse the conversion of bicarbonate, a blood pH buffer, into water and carbon dioxide, or can catalyse the reaction in the opposite direction when water and carbon dioxide are more abundant. Some reactions can even run in both directions depending on the concentration of molecules. A reaction can also be speeded by increasing the concentration of reactants, the chemicals that are necessary for the reaction to proceed this is called the Law of Mass Action, or by decreasing the concentration of products, the chemicals that result from the reaction. The same effect can be obtained by physically stirring the ingredients.
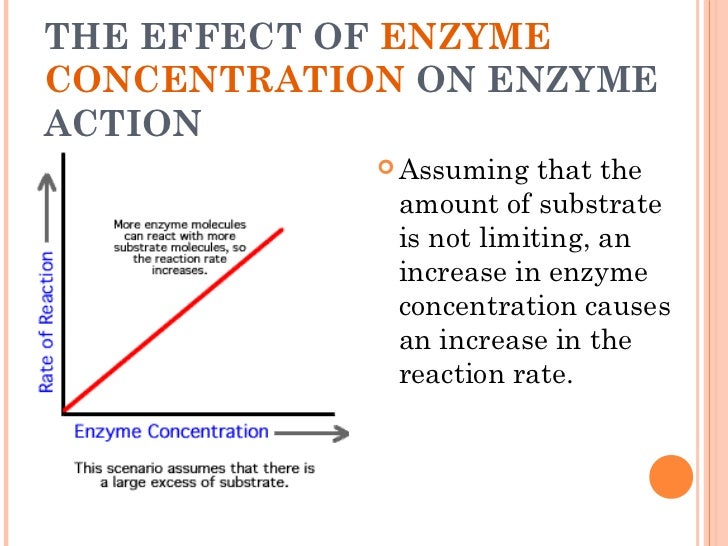
Raising the temperature can speed a reaction because the molecules have more energy and therefore bump into each other more frequently. There are several factors that can increase the rate of a reaction. Some examples of enzymes and their specific substrates. At very high temperatures, enzymes, because they are made of protein, can be denatured or destroyed. Outside of this zone, they are less effective. Enzymes are designed to work most effectively at a specific temperature and pH. In other words, they are not used up by the reaction and can be re-used.

Enzymes speed the reaction, or allow it to occur at lower energy levels and, once the reaction is complete, they are again available. In biology, chemical reactions are often aided by enzymes, biological molecules made of proteins which can be thought of as facilitators or catalysts. A good example is a lightning strike that starts a forest fire which, once started, will continue to burn until the fuel is used up. Once the activation energy is added, the reaction will continue if the final energy state is lower than the initial energy state. Other reactions require energy to get the reaction started.
For example, iron in the presence of oxygen will form iron oxide, or rust. Some reactions will occur just by putting two substances in close proximity. Chemical reactions occur when molecules interact and chemical bonds between them are formed or broken.


 0 kommentar(er)
0 kommentar(er)
